|
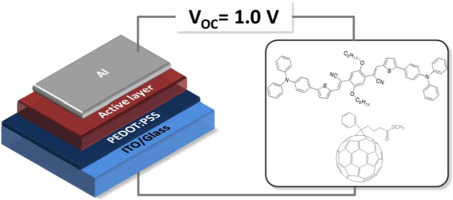
In our most recent publication, a donor-acceptor-donor (D-A-D) molecule has been designed and synthesized for use as the electron-donating material for organic solar cells. The D-A-D molecule (ZOPTAN-TPA) features a low HOMO level of −5.2 eV and an optical energy gap of 2.1 eV. Our best organic solar cell exhibited a power conversion efficiency of 1.9% and a high open-circuit voltage of 1.0 V. This work was developed together with the research groups of Prof. Bertil Eliasson, and Prof. Ludvig Edman. Javed Iqbal, Jenny Enevold, Christian Larsen, Jia Wang, Srikanth Revoju, Hamid Reza Barzegar, Thomas Wågberg, Bertil Eliasson, and Ludvig Edman. Solar Energy Materials and Solar Cells, 155, 348–355 (2016) DOI: 10.1016/j.solmat.2016.06.018 Abstract A donor-acceptor-donor (D-A-D) molecule has been designed and synthesized for use as the electron-donating material in solution-processed small-molecule organic solar cells (OSCs). The D-A-D molecule comprises a central electron-accepting (2Z,2′Z)-2,2′-(2,5-bis(octyloxy)-1,4-phenylene)bis(3-(thiophen-2-yl)acrylonitrile) (ZOPTAN) core, which is chemically connected to two peripheral and electron-donating triphenylamine (TPA) units. The ZOPTAN-TPA molecule features a low HOMO level of −5.2 eV and an optical energy gap of 2.1 eV. Champion OSCs based on a solution-processed and non-annealed active-material blend of [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) and ZOPTAN-TPA in a mass ratio of 2:1 exhibits a power conversion efficiency of 1.9% and a high open-circuit voltage of 1.0 V.
0 Comments
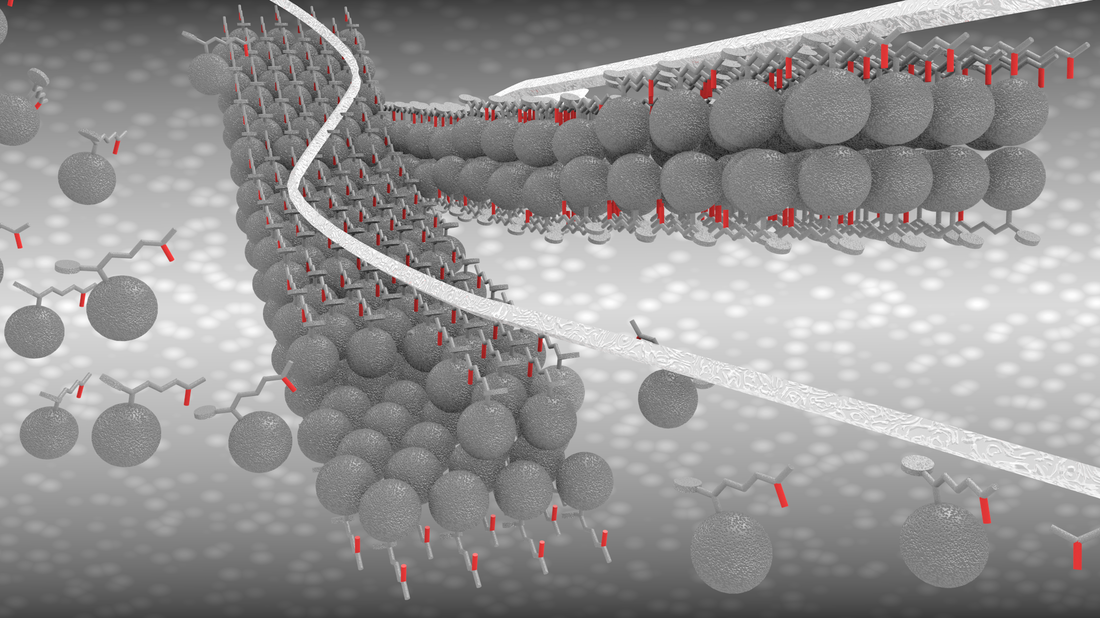
Our most recent work has been published in the journal of ACSnano. This time we synthesized one-dimensional zigzag PCBM nanoribbons that are ~4 nm wide, equivalent to four PCBM molecules, and lengths of 20–400 nm. These nanoribbons show well-defined crystalline structure, comprising PCBM molecules in a hexagonal arrangement. The manuscript has been published as open access and can be downloaded below.
AbstractOne-dimensional (1D) zigzag [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) nanoribbons are produced by folding two-dimensional ultrathin PCBM nanosheets in a simple solvent process. The unique 1D PCBM nanostructures exhibit uniform width of 3.8 ± 0.3 nm, equivalent to four PCBM molecules, and lengths of 20–400 nm. These nanoribbons show well-defined crystalline structure, comprising PCBM molecules in a hexagonal arrangement without trapped solvent molecules. First-principle calculations and detailed experimental characterization provide an insight into the structure and formation mechanism of the 1D PCBM nanoribbons. Given their dimensions and physical properties, we foresee that these nanostructures should be ideal as acceptor material in organic solar cells.
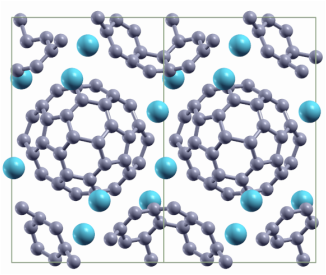
Here we performed calorimetric measurements of Na4C60 and Li4C60 crystals, the results are published in the Journal of Chemical Physics. Akira Inaba, Yuji Miyazaki, Paweł P. Michałowski, Eduardo Gracia-Espino, Bertil Sundqvist and Thomas Wågberg. J. Chem. Phys. 142, 164706 (2015) AbstractWe show specific heat data for Na4C60 and Li4C60 in the range 0.4-350 K for samples characterized by Raman spectroscopy and X-ray diffraction. At high temperatures, the two different polymer structures have very similar specific heats both in absolute values and in general trend. The specific heat data are compared with data for undoped polymeric and pristine C60. At high temperatures, a difference in specific heat between the intercalated and undoped C60 polymers of 100 J K−1 mol−1 is observed, in agreement with the Dulong-Petit law. At low temperatures, the specific heat data for Li4C60 and Na4C60 are modified by the stiffening of vibrational and librational molecular motion induced by the polymer bonds. The covalent twin bonds in Li4C60 affect these motions to a somewhat higher degree than the single intermolecular bonds in Na4C60. Below 1 K, the specific heats of both materials become linear in temperature, as expected from the effective dimensionality of the structure. The contribution to the total specific heat from the inserted metal ions can be well described by Einstein functions with T E = 386 K for Li4C60 and T E = 120 K for Na4C60, but for both materials we also observe a Schottky-type contribution corresponding to a first approximation to a two-level system with ΔE = 9.3 meV for Li4C60 and 3.1 meV for Na4C60, probably associated with jumps between closely spaced energy levels inside “octahedral-type” ionic sites. Static magnetic fields up to 9 T had very small effects on the specific heat below 10 K.
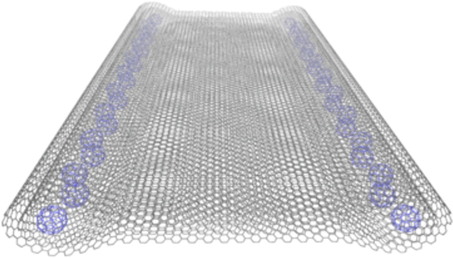
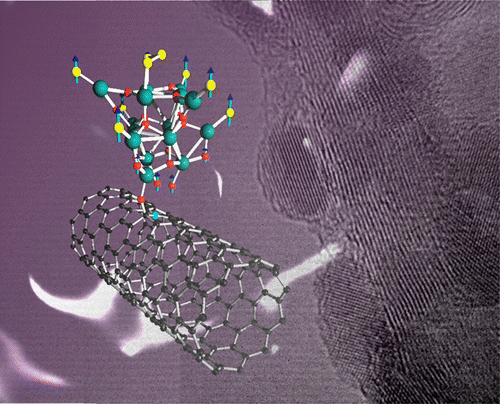
Our most recent work has been published in the journal of Nano letters. This time we introduced C60 fullerenes inside collapsed carbon nanotubes. The overfilling of the collapsed tubes allows a complete reinflation, where the C60 core shows a well defined crystalline structure. This work has been published as an open access, the file can be downloaded here. Hamid R. Barzegar, Eduardo Gracia-Espino, Aiming Yan, Claudia Ojeda-Aristizabal, Gabriel Dunn, Thomas Wågberg, and Alex Zettl. Nano letters 15, 829–834 (2015) AbstractWe examine a variant of so-called carbon nanotube peapods by packing C60 molecules inside the open edge ducts of collapsed carbon nanotubes. C60 insertion is accomplished through a facile single-step solution-based process. Theoretical modeling is used to evaluate favorable low-energy structural configurations. Overfilling of the collapsed tubes allows infiltration of C60 over the full cross-section of the tubes and consequent partial or complete reinflation, yielding few-wall, large diameter cylindrical nanotubes packed with crystalline C60 solid cores.
|
Nano for Energy group
Categories
All
Featured publications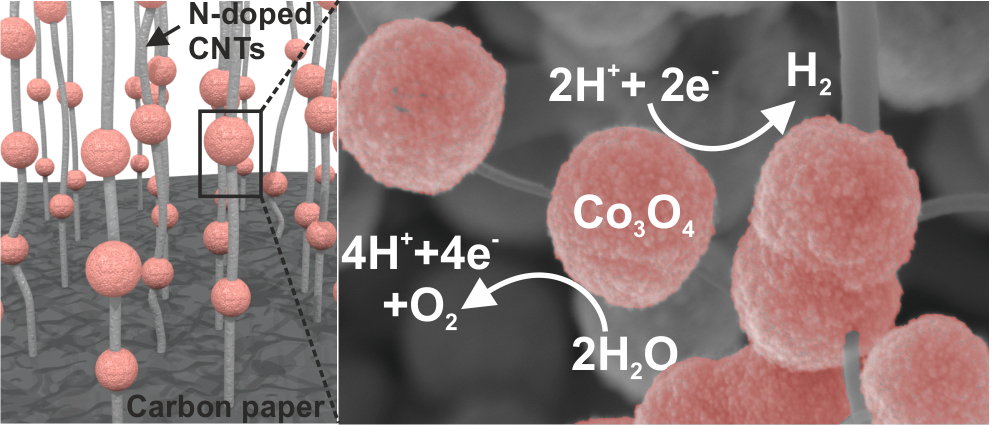
Comprehensive Study of an Earth-Abundant Bifunctional 3D Electrode for Efficient Water Electrolysis in Alkaline Medium.
ACS Appl. Mater. Interfaces, 2015, 7, 28148 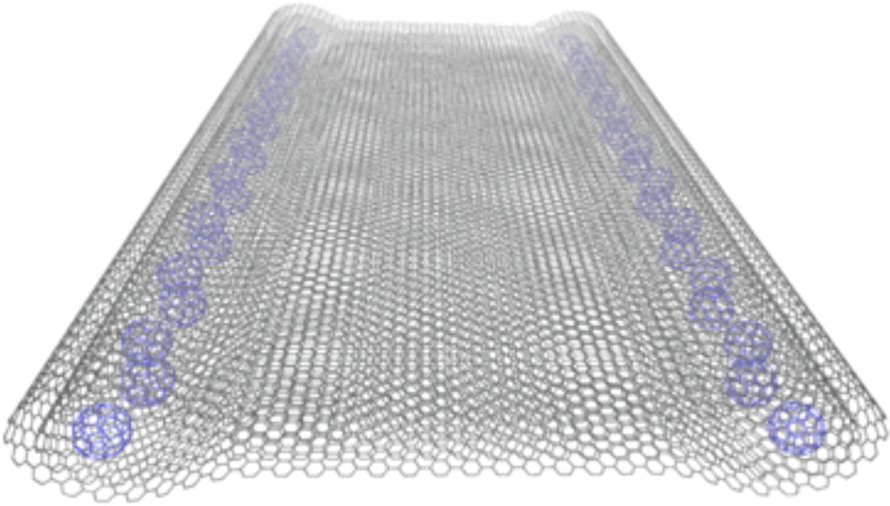
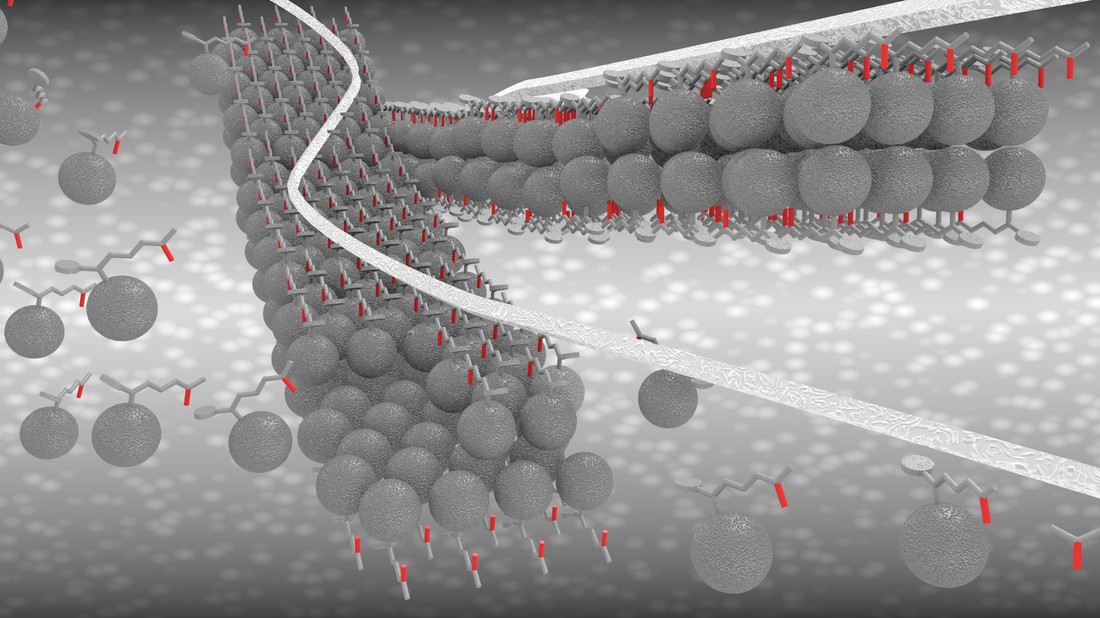
Fabrication of One-Dimensional Zigzag [6,6]-Phenyl-C61-Butyric Acid Methyl Ester Nanoribbons from Two-Dimensional Nanosheets.
ACS Nano, 2015, 9, 10516 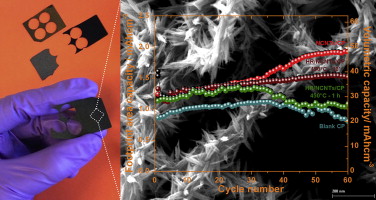
Hierarchical self-assembled structures based on nitrogen-doped carbon nanotubes as advanced negative electrodes for Li-ion batteries and 3D microbatteries.
J. P. Sources, 2015, 279, 581 .Self-Assembly Synthesis of Decorated Nitrogen-Doped Carbon Nanotubes with ZnO Nanoparticles: Anchoring Mechanism and the Effects of Sulfur.
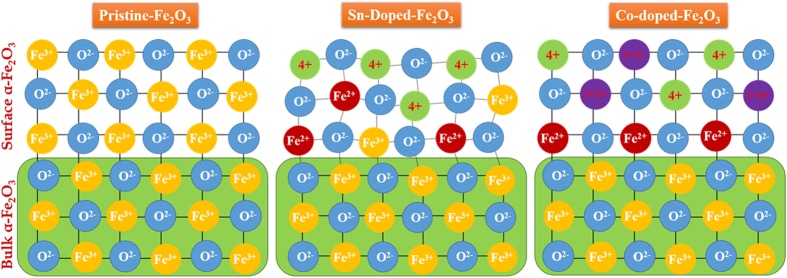
J. Phys. Chem. C, 120, 27849 (2016) Sn/Be Sequentially co-doped Hematite Photoanodes for Enhanced Photoelectrochemical Water Oxidation: Effect of Be2+ as co-dopant.
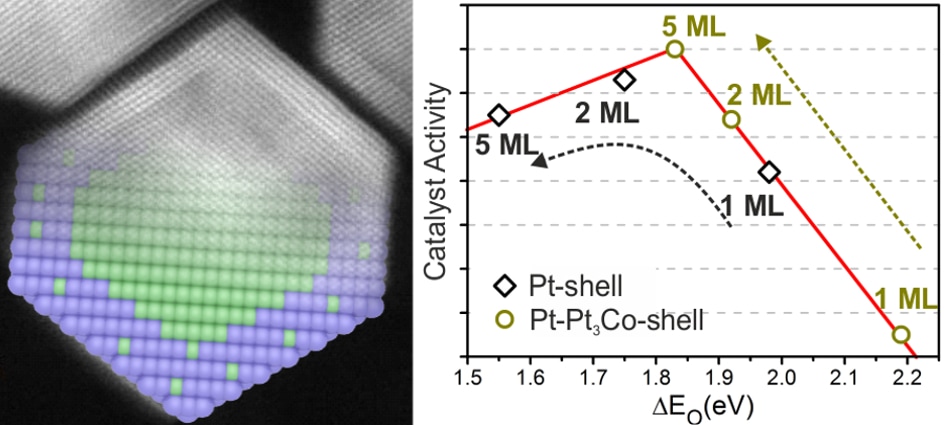
Sci Rep. 2016; 6: 23183. Atomistic understanding of the origin of high oxygen reduction electrocatalytic activity of cuboctahedral Pt3Co–Pt core–shell nanoparticles.
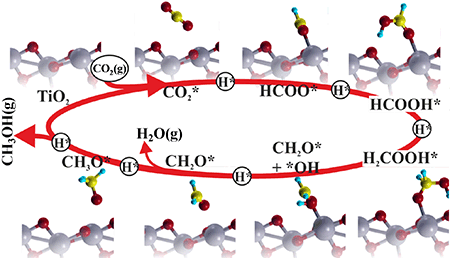
Catal. Sci. Technol., 2016, 6, 1393-1401 Photocatalytic reduction of CO2 with H2O over modified TiO2 nanofibers: Understanding the reduction pathway.
Nano Res. (2016) 9: 1956. |
 RSS Feed
RSS Feed