|
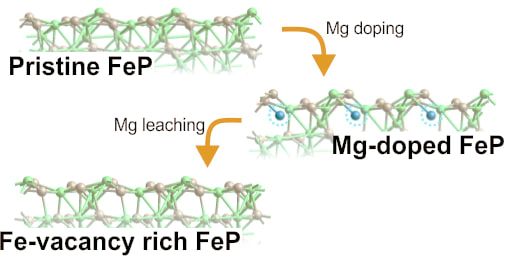
We created Fe vacancies as an approach to modulate the electronic structure and catalytic activity of iron phosphide (FeP). The Fe-vacancy-rich FeP nanoparticulate films showed excellent HER activity achieving a current density of 10 mA cm-2 at overpotentials of 108 mV in 1 M KOH, and 65 mV in 0.5 M H2SO4. This work was carried out in collaboration with Prof. Messinger (Uppsala University). W. L. Kwong, E. Gracia-Espino, C. C. Lee, R. Sandström, T. Wågberg, and J. Messinger. ChemSusChem (2017) DOI: 10.1002/cssc.201701565 AbstractEngineering the electronic properties of transition metal phosphides has shown great effectiveness in improving their intrinsic catalytic activity for the hydrogen evolution reaction (HER) in water splitting applications. Herein, we report for the first time, the creation of Fe vacancies as an approach to modulate the electronic structure of iron phosphide (FeP). The Fe vacancies were produced via chemical leaching of Mg that was introduced into FeP as 'sacrificial dopant'. The obtained Fe-vacancy-rich FeP nanoparticulate films, which were deposited on Ti foil, shows excellent HER activity as compared to pristine FeP and Mg-doped FeP, achieving a current density of 10 mA cm-2 at overpotentials of 108 mV in 1 M KOH and 65 mV in 0.5 M H2SO4, with a near-100% Faradaic efficiency. Our theoretical and experimental analyses reveal that the improved HER activity originates from the presence of Fe vacancies, which lead to a synergistic modulation of the structural and electronic properties that result in a near optimal hydrogen adsorption free energy and enhanced proton trapping. The success in catalytic improvement via the introduction of cationic vacancy defects has not only demonstrated the potential of Fe-vacancy-rich FeP as highly efficient, earth abundant HER catalyst, but also opened up an exciting pathway for activating other promising catalysts for electrochemical water splitting.
0 Comments
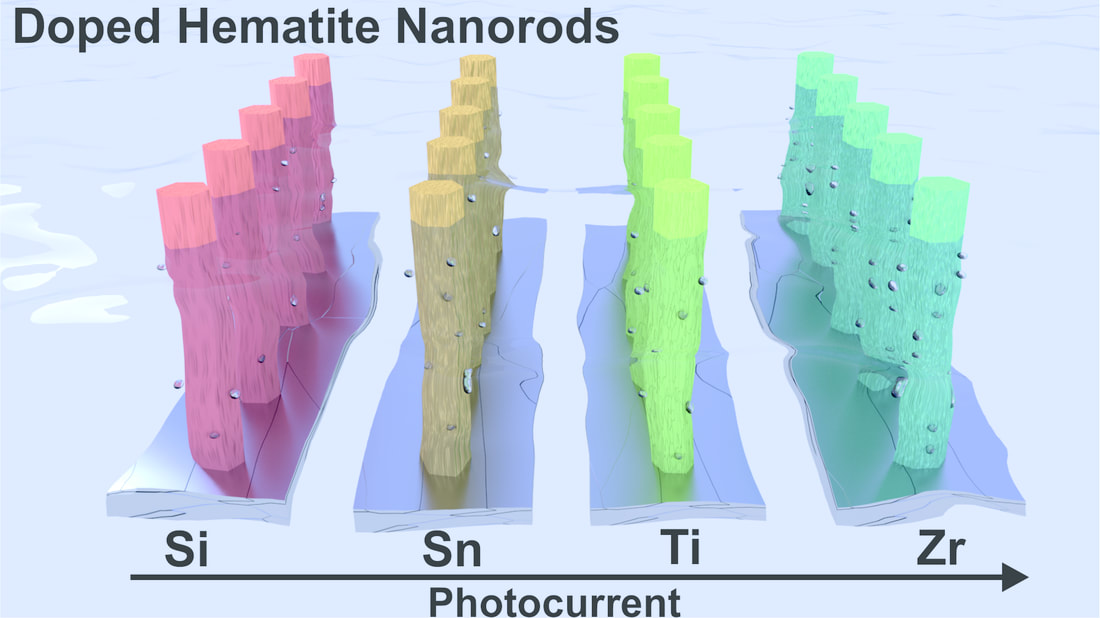
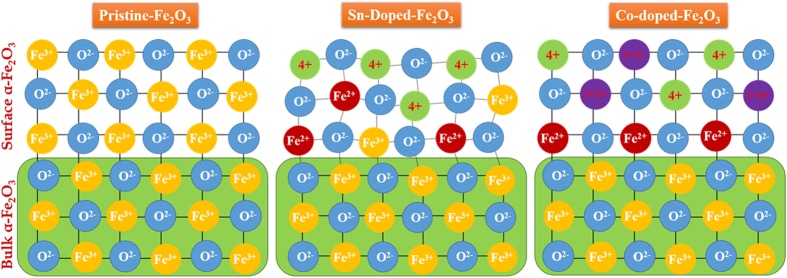
This time the influence of tetravalent dopants such as Si4+, Sn4+, Ti4+, and Zr4+ on hematite nanostructure for enhanced photoelectrochemical water splitting is reported. The photoactivity of the doped photoanodes at 1.23 V RHE follows the order Zr > Sn > Ti > Si. The work was performed in collaboration with Prof. Jum Suk Jang (Chonbuk National University, Korea), and the results are published in the journal of Applied Surface Science. A. Subramanian, E. Gracia-Espino, A. Annamalai, H. H. Lee, S Y. Lee, S. H. Choi, and J. S. Jang. Applied Surface Science (2017). DOI: 10.1016/j.apsusc.2017.09.042 Abstract In this paper, the influence of tetravalent dopants such as Si4+, Sn4+, Ti4+, and Zr4+ on the hematite (α-Fe2O3) nanostructure for enhanced photoelectrochemical (PEC) water splitting are reported. The tetravalent doping was performed on hydrothermally grown akaganeite (β-FeOOH) nanorods on FTO (fluorine-doped tin-oxide) substrates via a simple dipping method for which the respective metal-precursor solution was used, followed by a high-temperature (800° C) sintering in a box furnace. The photocurrent density for the pristine (hematite) photoanode is ∼0.81 mA/cm2 at 1.23 VRHE, with an onset potential of 0.72 VRHE; however, the tetravalent dopants on the hematite nanostructures alter the properties of the pristine photoanode. The Si4+-doped hematite photoanode showed a slight photocurrent increment without a changing of the onset potential of the pristine photoanode. The Sn4+- and Ti4+-doped hematite photoanodes, however, showed an anodic shift of the onset potential with the photocurrent increment at a higher applied potential. Interestingly, the Zr4+-doped hematite photoanode exhibited an onset potential that is similar to those of the pristine and Si4+-doped hematite, but a larger photocurrent density that is similar to those of the Sn4+- and Ti4+-doped photoanodes was recorded. The photoactivity of the doped photoanodes at 1.23 VRHE follows the order Zr > Sn > Ti > Si. The onset-potential shifts of the doped photoanodes were investigated using the Ab initio calculations that are well correlated with the experimental data. X-ray diffraction (XRD) and scanning-electron microscopy (FESEM) revealed that both the crystalline phase of the hematite and the nanorod morphology were preserved after the doping procedure. X-ray photoelectron spectroscopy (XPS) confirmed the presence of the tetravalent dopants on the hematite nanostructure. The charge-transfer resistance at the various interfaces of the doped photoanodes was studied using impedance spectroscopy. The doping on the hematite photoanodes was confirmed using the Mott-Schottky (MS) analysis.
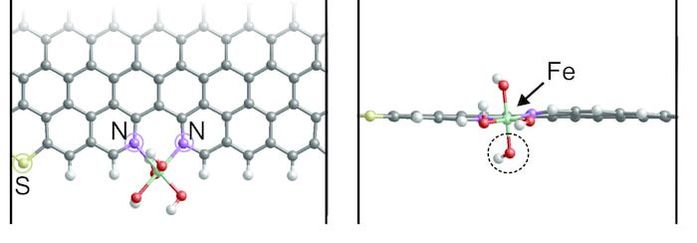
In this occasion, we investigated a sulfur-doped Fe-N-C (Fe/SNC) catalyst with a thiophene-like structure (C-S-C) that reduces the electron localization around the Fe center and improves the interaction with oxygenated species. The observed synergistic effect makes the Fe/SNC catalyst exhibits better ORR activity than sulfur free catalyst (Fe/NC) in 0.5 M H2SO4. The results were published in the journal of Angewandte Chemie International Edition. Hangjia Shen, Eduardo Gracia- Espino, Jingyuan Ma, Ketao Zang, Jun Luo, Le Wang, Sanshuang Gao, Xamxikamar Mamat, Guangzhi Hu, Thomas Wagberg, and Shaojun Guo. Angew. Chem. Int. Ed. (2017), DOI: 10.1002/anie.201706602 AbstractVarious advanced catalysts of sulfur doped Fe-N-C materials have been recently designed for oxygen reduction reaction (ORR), however, the enhanced activity is still controversial and usually attributed to differences in surface area, improved conductivity, or to uncertain synergistic effects. Here, a sulfur-doped Fe-N-C catalyst (denoted as Fe/SNC) derived via a template sacrificing method is presented. The incorporated S gives a thiophene-like structure (C-S-C), reduces the electron localization around the Fe center, improves the interaction with oxygenated species, and therefore facilitates the complete 4e- ORR in acid solution. This synergistic effect makes the Fe/SNC catalyst exhibits much better ORR activity than sulfur free catalyst (Fe/NC) in 0.5 M H2SO4.
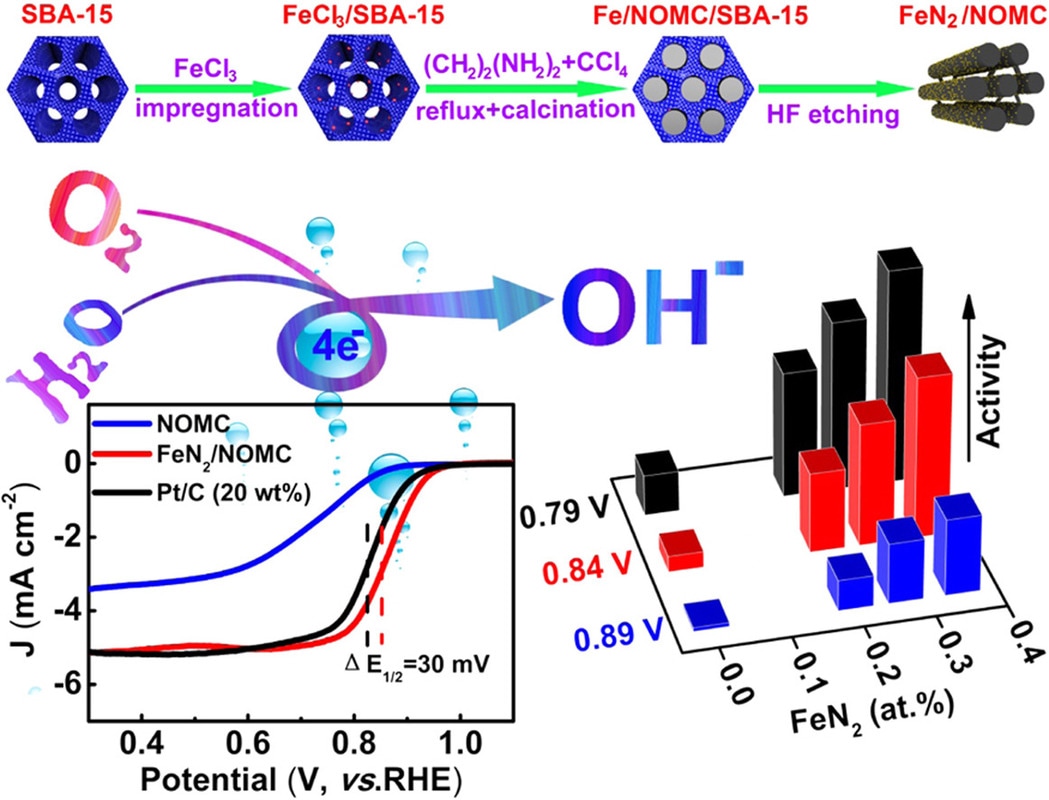
We now report a template casting strategy to easily introduce atomically dispersed FeN2 moieties onto the surface of N-doped ordered mesoporous carbon with extraordinary catalytic activity towards ORR. This work was carried out in collaboration with Prof. Guo (College of Engineering, Peking University) and Prof. Hu (Xinjiang Technical Institute of Physics and Chemistry). The results are published in the journal of Nano Energy. H. Shena, E. Gracia-Espino, J. Mac, H. Tang, X. Mamat, T. Wagberg, G. Hua, S. Guoe. Nano Energy, (2017) DOI: 10.1016/j.nanoen.2017.03.027 Abstract Earth-abundant materials with Fe-N-C centers have been identified as promising catalysts for oxygen reduction reaction (ORR), but these alternatives for Pt catalysts are usually the porphyrin-like FeN4 configuration. The density functional theory (DFT) calculations reveal that FeN2 outperforms FeN4 due to its lower interaction with ⁎O2 and ⁎OH intermediates and enhanced electron transport, however, achieving an optimum design of these earth-abundant materials with the enriched FeN2 catalytic centers is still a great challenge. Here, we report an intriguing template casting strategy to introduce a mass of atomically dispersed FeN2 moieties onto the surface of N-doped ordered mesoporous carbon for boosting ORR electrocatalysis. One of unique parts herein is to pre anchor Fe precursor on the surface of template (SBA-15) during catalyst synthesis, preventing Fe from penetrating into the carbon skeleton and facilitating the removal of excessive Fe-based particles during silica elimination by HF etching, resulting in a desirable model structure comprising only highly active atomically dispersed FeN2 sites, as confirmed by high-angle annular dark field-scanning transmission electron microscopy (HAADF-STEM), extended X-ray absorption fine structure (EXAFS) and Mößbauer spectroscopy analysis. The well-defined structure prompts us to understand the nature of the catalytic active sites, and to demonstrate that the catalyst activity is linearly proportional to the concentration of FeN2 sites. The obtained atomic electrocatalyst exhibits superior electrocatalytic performance for ORR with a more positive half-wave potential than that of Pt/C catalyst. We further establish a kinetic model to predict the ORR activity of these single-atom dispersed catalysts. The present work elaborates on a profound understanding for designing low-cost, highly efficient FeN2-based electrocatalyst for boosting ORR.
|
Nano for Energy group
Categories
All
Featured publications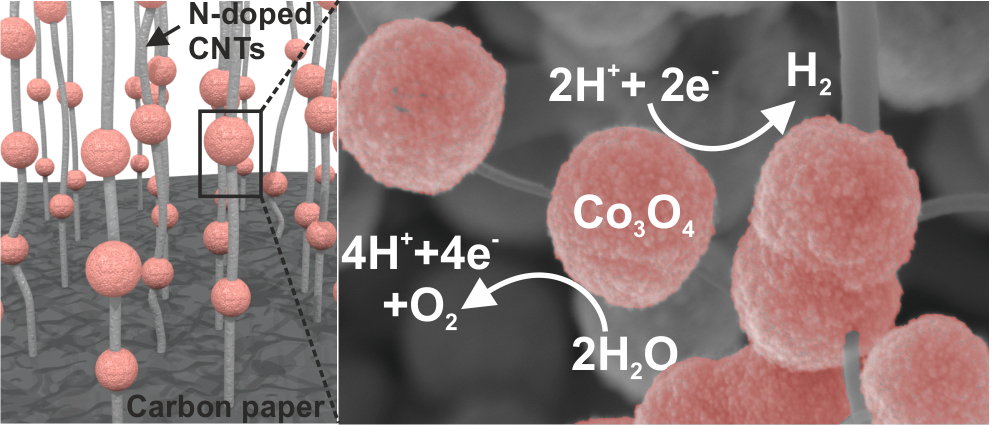
Comprehensive Study of an Earth-Abundant Bifunctional 3D Electrode for Efficient Water Electrolysis in Alkaline Medium.
ACS Appl. Mater. Interfaces, 2015, 7, 28148 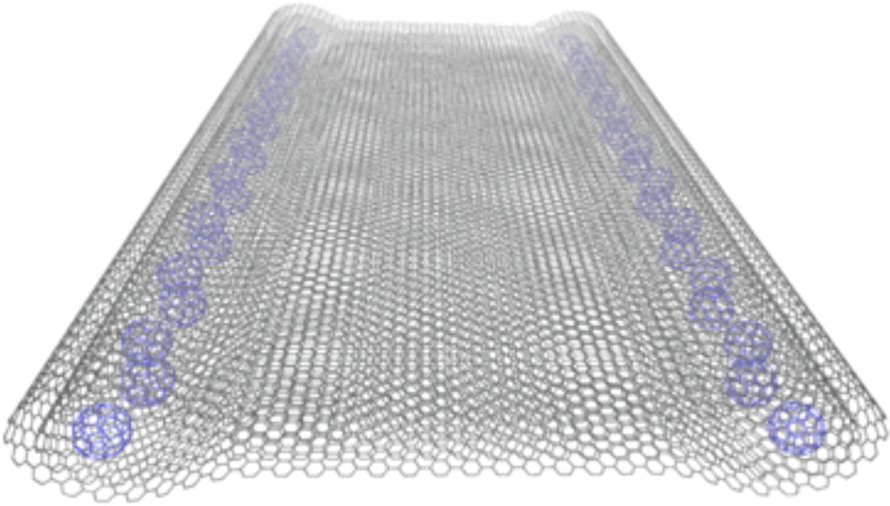
Fabrication of One-Dimensional Zigzag [6,6]-Phenyl-C61-Butyric Acid Methyl Ester Nanoribbons from Two-Dimensional Nanosheets.
ACS Nano, 2015, 9, 10516 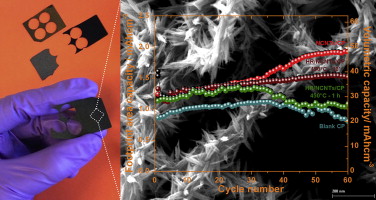
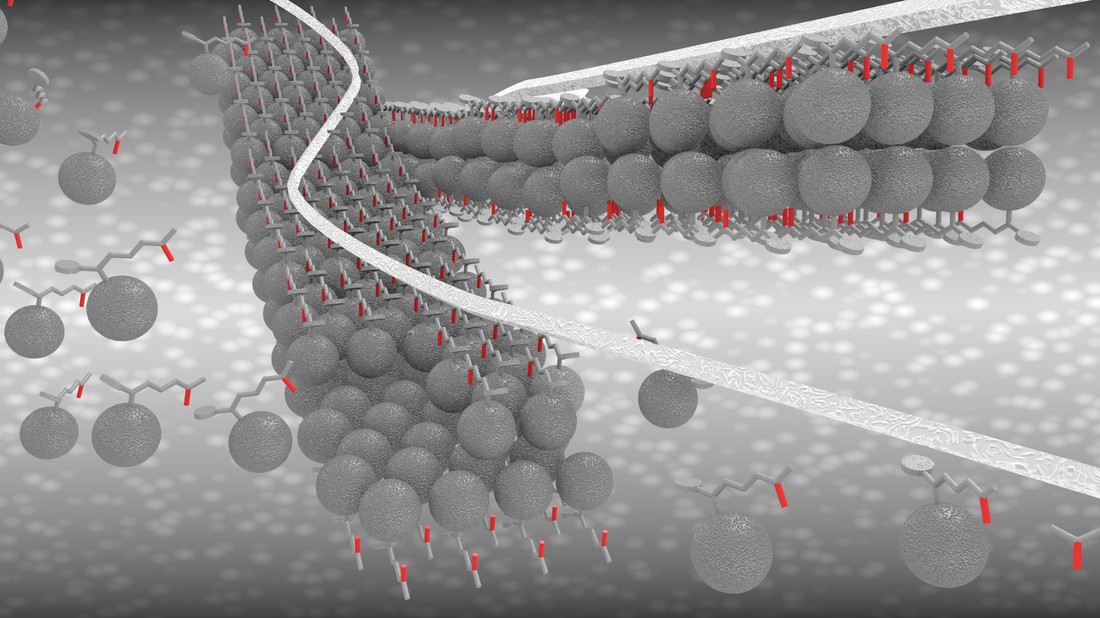
Hierarchical self-assembled structures based on nitrogen-doped carbon nanotubes as advanced negative electrodes for Li-ion batteries and 3D microbatteries.
J. P. Sources, 2015, 279, 581 .Self-Assembly Synthesis of Decorated Nitrogen-Doped Carbon Nanotubes with ZnO Nanoparticles: Anchoring Mechanism and the Effects of Sulfur.
J. Phys. Chem. C, 120, 27849 (2016) Sn/Be Sequentially co-doped Hematite Photoanodes for Enhanced Photoelectrochemical Water Oxidation: Effect of Be2+ as co-dopant.
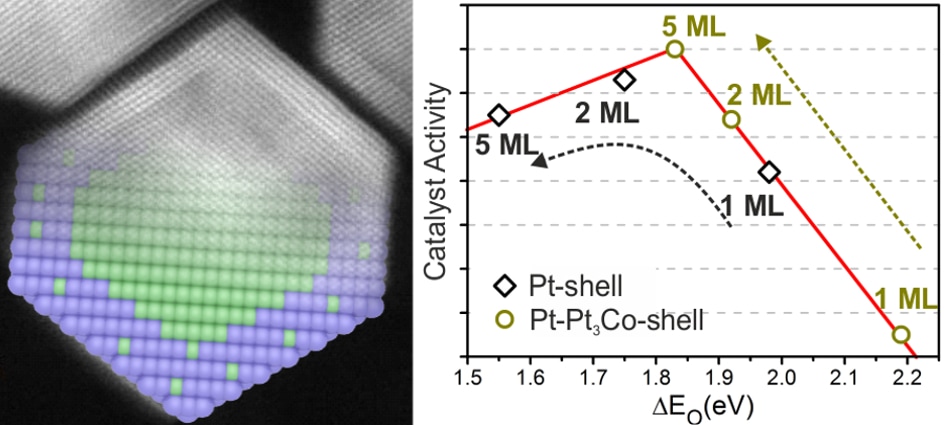
Sci Rep. 2016; 6: 23183. Atomistic understanding of the origin of high oxygen reduction electrocatalytic activity of cuboctahedral Pt3Co–Pt core–shell nanoparticles.
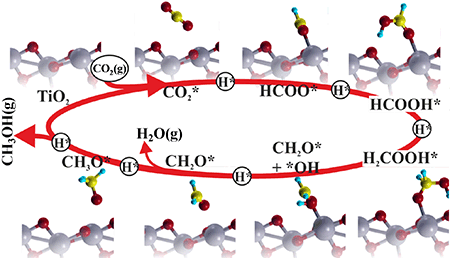
Catal. Sci. Technol., 2016, 6, 1393-1401 Photocatalytic reduction of CO2 with H2O over modified TiO2 nanofibers: Understanding the reduction pathway.
Nano Res. (2016) 9: 1956. |
 RSS Feed
RSS Feed