|
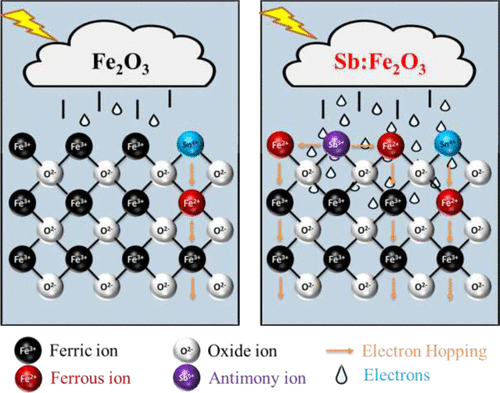
We report the production of antimony (Sb5+) doped hematite photoanodes by a simple ex situ method. The Sb5+ dopant acts as effective electron donor, reduces recombination effects and improves the surface wettability. In addition, the presence of Sb5+ states in Sb-doped Fe2O3 photoanodes exhibit a 10-fold increase in carrier concentration and decreased photoanode/electrolyte charge transfer resistance. A. Annamalai, R. Sandström, E. Gracia-Espino, N. Boulanger, J.-F. Boily, I. Mühlbacher, A. Shchukarev, T. Wågberg. ACS Appl. Mater. Interfaces. DOI: 10.1021/acsami.8b02147 AbstractTo exploit the full potential of hematite (α-Fe2O3) as an efficient photoanode for water oxidation, the redox processes occurring at the Fe2O3/electrolyte interface need to be studied in greater detail. Ex situ doping is an excellent technique to introduce dopants onto the photoanode surface and to modify the photoanode/electrolyte interface. In this context, we selected antimony (Sb5+) as the ex situ dopant because it is an effective electron donor and reduces recombination effects and concurrently utilize the possibility to tuning the surface charge and wettability. In the presence of Sb5+ states in Sb-doped Fe2O3 photoanodes, as confirmed by X-ray photoelectron spectroscopy, we observed a 10-fold increase in carrier concentration (1.1 × 1020 vs 1.3 × 1019 cm–3) and decreased photoanode/electrolyte charge transfer resistance (∼990 vs ∼3700 Ω). Furthermore, a broad range of surface characterization techniques such as Fourier-transform infrared spectroscopy, ζ-potential, and contact angle measurements reveal that changes in the surface hydroxyl groups following the ex situ doping also have an effect on the water splitting capability. Theoretical calculations suggest that Sb5+ can activate multiple Fe3+ ions simultaneously, in addition to increasing the surface charge and enhancing the electron/hole transport properties. To a greater extent, the Sb5+- surface-doped determines the interfacial properties of electrochemical charge transfer, leading to an efficient water oxidation mechanism.
0 Comments
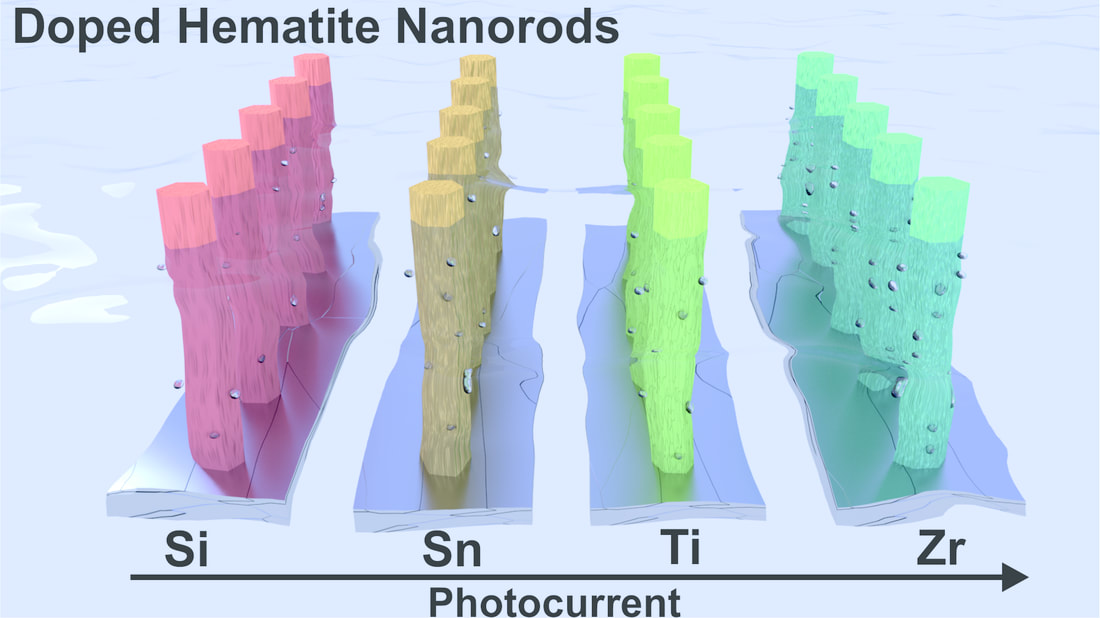
This time the influence of tetravalent dopants such as Si4+, Sn4+, Ti4+, and Zr4+ on hematite nanostructure for enhanced photoelectrochemical water splitting is reported. The photoactivity of the doped photoanodes at 1.23 V RHE follows the order Zr > Sn > Ti > Si. The work was performed in collaboration with Prof. Jum Suk Jang (Chonbuk National University, Korea), and the results are published in the journal of Applied Surface Science. A. Subramanian, E. Gracia-Espino, A. Annamalai, H. H. Lee, S Y. Lee, S. H. Choi, and J. S. Jang. Applied Surface Science (2017). DOI: 10.1016/j.apsusc.2017.09.042 Abstract In this paper, the influence of tetravalent dopants such as Si4+, Sn4+, Ti4+, and Zr4+ on the hematite (α-Fe2O3) nanostructure for enhanced photoelectrochemical (PEC) water splitting are reported. The tetravalent doping was performed on hydrothermally grown akaganeite (β-FeOOH) nanorods on FTO (fluorine-doped tin-oxide) substrates via a simple dipping method for which the respective metal-precursor solution was used, followed by a high-temperature (800° C) sintering in a box furnace. The photocurrent density for the pristine (hematite) photoanode is ∼0.81 mA/cm2 at 1.23 VRHE, with an onset potential of 0.72 VRHE; however, the tetravalent dopants on the hematite nanostructures alter the properties of the pristine photoanode. The Si4+-doped hematite photoanode showed a slight photocurrent increment without a changing of the onset potential of the pristine photoanode. The Sn4+- and Ti4+-doped hematite photoanodes, however, showed an anodic shift of the onset potential with the photocurrent increment at a higher applied potential. Interestingly, the Zr4+-doped hematite photoanode exhibited an onset potential that is similar to those of the pristine and Si4+-doped hematite, but a larger photocurrent density that is similar to those of the Sn4+- and Ti4+-doped photoanodes was recorded. The photoactivity of the doped photoanodes at 1.23 VRHE follows the order Zr > Sn > Ti > Si. The onset-potential shifts of the doped photoanodes were investigated using the Ab initio calculations that are well correlated with the experimental data. X-ray diffraction (XRD) and scanning-electron microscopy (FESEM) revealed that both the crystalline phase of the hematite and the nanorod morphology were preserved after the doping procedure. X-ray photoelectron spectroscopy (XPS) confirmed the presence of the tetravalent dopants on the hematite nanostructure. The charge-transfer resistance at the various interfaces of the doped photoanodes was studied using impedance spectroscopy. The doping on the hematite photoanodes was confirmed using the Mott-Schottky (MS) analysis.
This time we report an artificial-leaf device where NiCo2O4 nanorods are used as bifunctional electrode that it can operate as both anode and cathode in the same alkaline solution. By driving two such identical electrodes with a perovskite photovoltaic assembly, a wired artificial-leaf device is obtained that features a Faradaic H2 evolution efficiency of 100%, and a solar-to-hydrogen conversion efficiency of 6.2%. This work was carried out in close collaboration with the research groups of Prof. Edman and Prof. Messinger. The article is published in the journal of Advanced Energy Materials as open access.
AbstractMolecular hydrogen can be generated renewably by water splitting with an “artificial-leaf device”, which essentially comprises two electrocatalyst electrodes immersed in water and powered by photovoltaics. Ideally, this device should operate efficiently and be fabricated with cost-efficient means using earth-abundant materials. Here, a lightweight electrocatalyst electrode, comprising large surface-area NiCo2O4 nanorods that are firmly anchored onto a carbon–paper current collector via a dense network of nitrogen-doped carbon nanotubes is presented. This electrocatalyst electrode is bifunctional in that it can efficiently operate as both anode and cathode in the same alkaline solution, as quantified by a delivered current density of 10 mA cm−2 at an overpotential of 400 mV for each of the oxygen and hydrogen evolution reactions. By driving two such identical electrodes with a solution-processed thin-film perovskite photovoltaic assembly, a wired artificial-leaf device is obtained that features a Faradaic H2 evolution efficiency of 100%, and a solar-to-hydrogen conversion efficiency of 6.2%. A detailed cost analysis is presented, which implies that the material-payback time of this device is of the order of 100 days.
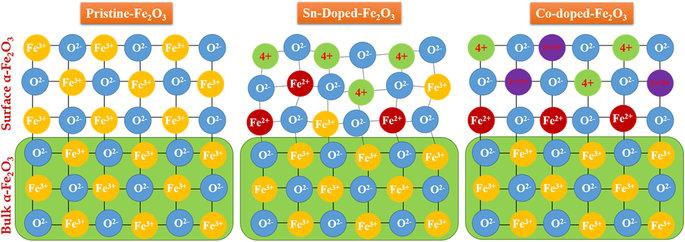
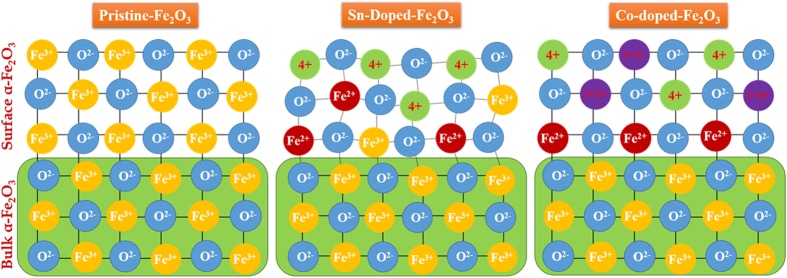
We report an ex-situ co-doping methods that enables rapid diffusion of Sn4+ and Be2+ dopants into hematite (α–Fe2O3) lattices without altering the nanorod morphology. Sn/Be co-doping results in a remarkable enhancement in photocurrent (1.7 mA/cm2) compared to pristine α–Fe2O3 (0.7 mA/cm2). The sequence in which the ex-situ co-doping is carried out is very crucial, as Be/Sn co-doping sequence induces many under-coordinated O atoms resulting in a higher micro-strain and lower charge separation efficiency resulting undesired electron recombination. These results are published in the journal of Scientific Reports.
Abstract For ex-situ co-doping methods, sintering at high temperatures enables rapid diffusion of Sn4+ and Be2+ dopants into hematite (α–Fe2O3) lattices, without altering the nanorod morphology or damaging their crystallinity. Sn/Be co-doping results in a remarkable enhancement in photocurrent (1.7 mA/cm2) compared to pristine α–Fe2O3 (0.7 mA/cm2), and Sn4+ mono-doped α-Fe2O3 photoanodes (1.0 mA/cm2). From first-principles calculations, we found that Sn4+ doping induced a shallow donor level below the conduction band minimum, which does not contribute to increase electrical conductivity and photocurrent because of its localized nature. Additionally, Sn4+-doping induce local micro-strain and a decreased Fe-O bond ordering. When Be2+ was co-doped with Sn4+-doped α–Fe2O3 photoanodes, the conduction band recovered its original state, without localized impurities peaks, also a reduction in micro-strain and increased Fe-O bond ordering is observed. Also the sequence in which the ex-situ co-doping is carried out is very crucial, as Be/Sn co-doping sequence induces many under-coordinated O atoms resulting in a higher micro-strain and lower charge separation efficiency resulting undesired electron recombination. Here, we perform a detailed systematic characterization using XRD, FESEM, XPS and comprehensive electrochemical and photoelectrochemical studies, along with sophisticated synchrotron diffraction studies and extended X-ray absorption fine structure.
|
Nano for Energy group
Categories
All
Featured publications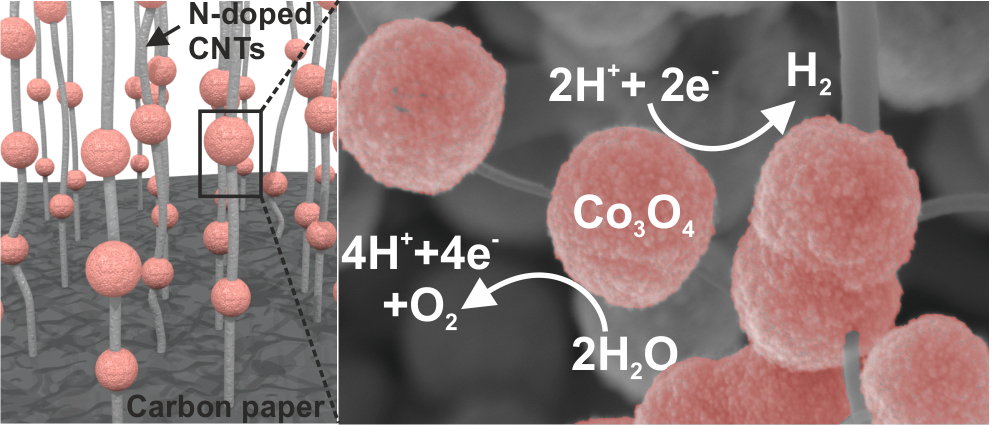
Comprehensive Study of an Earth-Abundant Bifunctional 3D Electrode for Efficient Water Electrolysis in Alkaline Medium.
ACS Appl. Mater. Interfaces, 2015, 7, 28148 
Fabrication of One-Dimensional Zigzag [6,6]-Phenyl-C61-Butyric Acid Methyl Ester Nanoribbons from Two-Dimensional Nanosheets.
ACS Nano, 2015, 9, 10516 
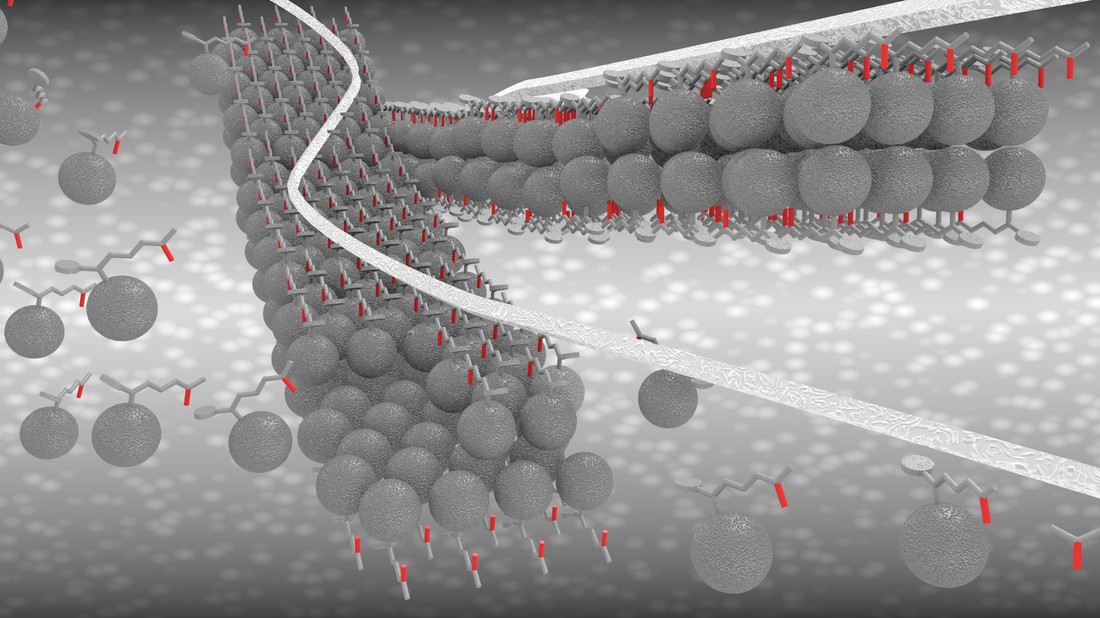
Hierarchical self-assembled structures based on nitrogen-doped carbon nanotubes as advanced negative electrodes for Li-ion batteries and 3D microbatteries.
J. P. Sources, 2015, 279, 581 .Self-Assembly Synthesis of Decorated Nitrogen-Doped Carbon Nanotubes with ZnO Nanoparticles: Anchoring Mechanism and the Effects of Sulfur.
J. Phys. Chem. C, 120, 27849 (2016) Sn/Be Sequentially co-doped Hematite Photoanodes for Enhanced Photoelectrochemical Water Oxidation: Effect of Be2+ as co-dopant.
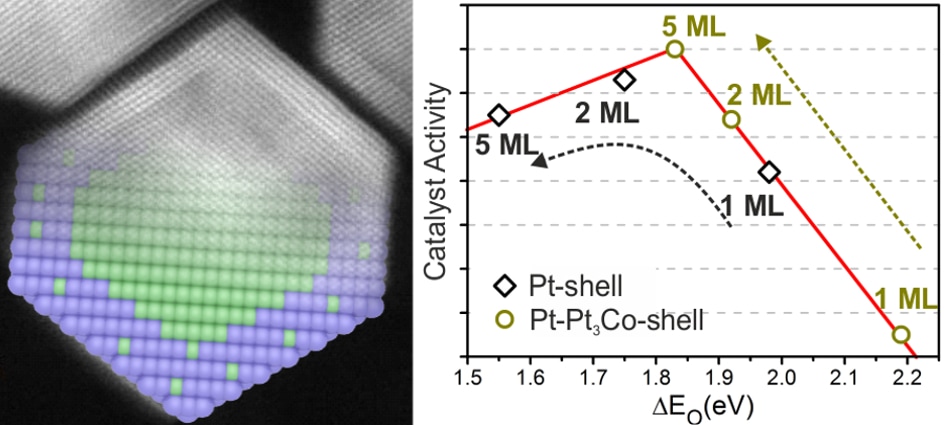
Sci Rep. 2016; 6: 23183. Atomistic understanding of the origin of high oxygen reduction electrocatalytic activity of cuboctahedral Pt3Co–Pt core–shell nanoparticles.
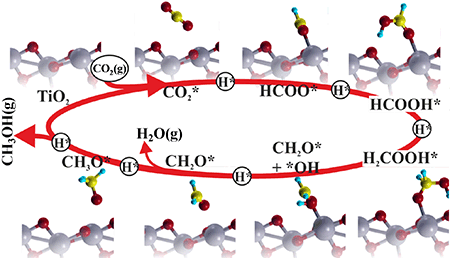
Catal. Sci. Technol., 2016, 6, 1393-1401 Photocatalytic reduction of CO2 with H2O over modified TiO2 nanofibers: Understanding the reduction pathway.
Nano Res. (2016) 9: 1956. |
 RSS Feed
RSS Feed