|
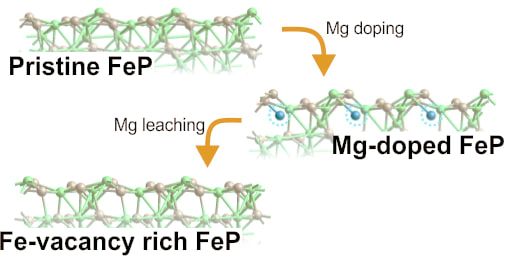
We created Fe vacancies as an approach to modulate the electronic structure and catalytic activity of iron phosphide (FeP). The Fe-vacancy-rich FeP nanoparticulate films showed excellent HER activity achieving a current density of 10 mA cm-2 at overpotentials of 108 mV in 1 M KOH, and 65 mV in 0.5 M H2SO4. This work was carried out in collaboration with Prof. Messinger (Uppsala University). W. L. Kwong, E. Gracia-Espino, C. C. Lee, R. Sandström, T. Wågberg, and J. Messinger. ChemSusChem (2017) DOI: 10.1002/cssc.201701565 AbstractEngineering the electronic properties of transition metal phosphides has shown great effectiveness in improving their intrinsic catalytic activity for the hydrogen evolution reaction (HER) in water splitting applications. Herein, we report for the first time, the creation of Fe vacancies as an approach to modulate the electronic structure of iron phosphide (FeP). The Fe vacancies were produced via chemical leaching of Mg that was introduced into FeP as 'sacrificial dopant'. The obtained Fe-vacancy-rich FeP nanoparticulate films, which were deposited on Ti foil, shows excellent HER activity as compared to pristine FeP and Mg-doped FeP, achieving a current density of 10 mA cm-2 at overpotentials of 108 mV in 1 M KOH and 65 mV in 0.5 M H2SO4, with a near-100% Faradaic efficiency. Our theoretical and experimental analyses reveal that the improved HER activity originates from the presence of Fe vacancies, which lead to a synergistic modulation of the structural and electronic properties that result in a near optimal hydrogen adsorption free energy and enhanced proton trapping. The success in catalytic improvement via the introduction of cationic vacancy defects has not only demonstrated the potential of Fe-vacancy-rich FeP as highly efficient, earth abundant HER catalyst, but also opened up an exciting pathway for activating other promising catalysts for electrochemical water splitting.
0 Comments
Your comment will be posted after it is approved.
Leave a Reply. |
Nano for Energy group
Categories
All
Featured publications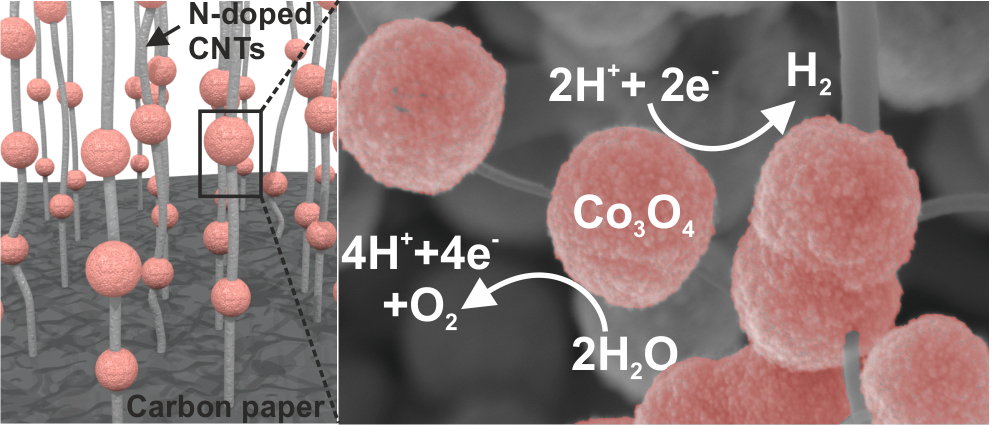
Comprehensive Study of an Earth-Abundant Bifunctional 3D Electrode for Efficient Water Electrolysis in Alkaline Medium.
ACS Appl. Mater. Interfaces, 2015, 7, 28148 
Fabrication of One-Dimensional Zigzag [6,6]-Phenyl-C61-Butyric Acid Methyl Ester Nanoribbons from Two-Dimensional Nanosheets.
ACS Nano, 2015, 9, 10516 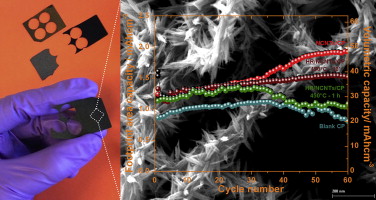
Hierarchical self-assembled structures based on nitrogen-doped carbon nanotubes as advanced negative electrodes for Li-ion batteries and 3D microbatteries.
J. P. Sources, 2015, 279, 581 .Self-Assembly Synthesis of Decorated Nitrogen-Doped Carbon Nanotubes with ZnO Nanoparticles: Anchoring Mechanism and the Effects of Sulfur.
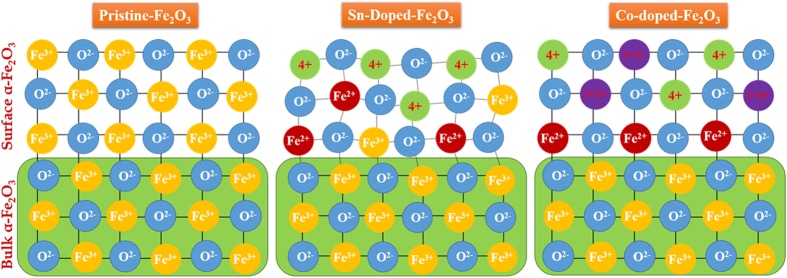
J. Phys. Chem. C, 120, 27849 (2016) Sn/Be Sequentially co-doped Hematite Photoanodes for Enhanced Photoelectrochemical Water Oxidation: Effect of Be2+ as co-dopant.
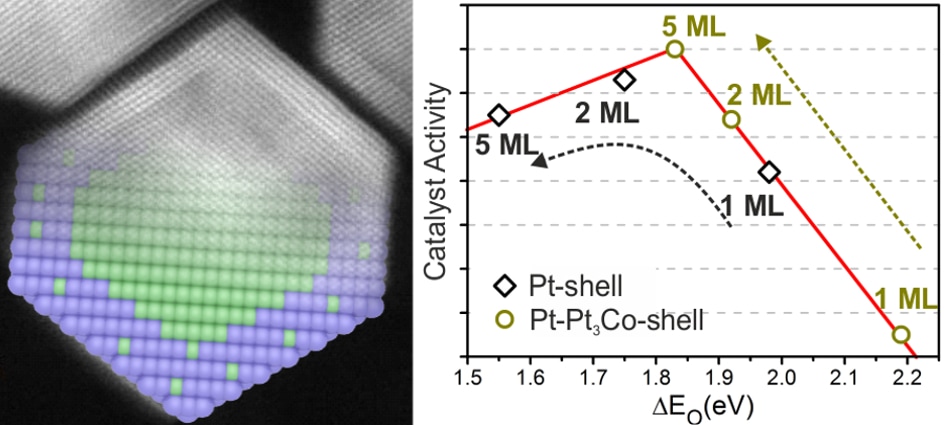
Sci Rep. 2016; 6: 23183. Atomistic understanding of the origin of high oxygen reduction electrocatalytic activity of cuboctahedral Pt3Co–Pt core–shell nanoparticles.
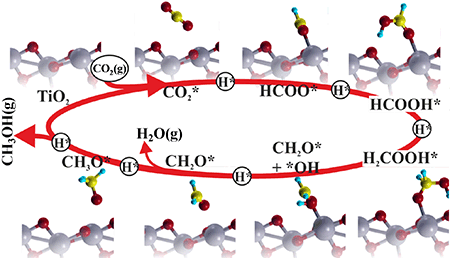
Catal. Sci. Technol., 2016, 6, 1393-1401 Photocatalytic reduction of CO2 with H2O over modified TiO2 nanofibers: Understanding the reduction pathway.
Nano Res. (2016) 9: 1956. |
 RSS Feed
RSS Feed