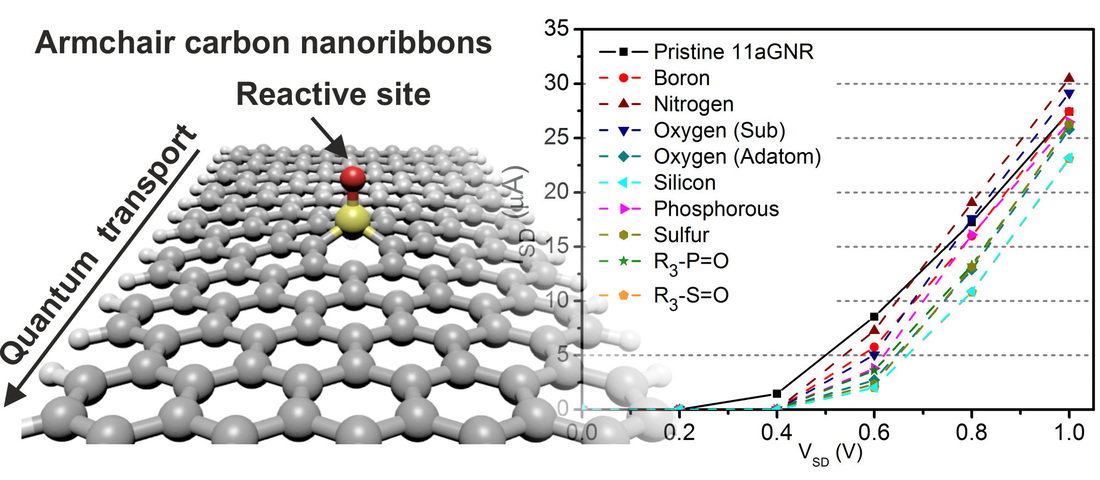
Electron transport study on functionalized armchair graphene nanoribbons: DFT calculations2/24/2016 Here we performed quantum transport studies on doped and functionalized 8- and 11-armchair graphene nanoribbons (aGNRs) by means of density functional theory. We introduced B, N, O, Si, P, and S within the lattice of the aGNRs. Other functional groups such as borane, amine, hydroxyl, thiol, silane, silene, phosphine, and phosphorane are also introduced at the nanoribbon's edge. Our results suggest that wider graphene nanoribbons could be functionalized at the inner sections without significantly compromising their transport characteristics while retaining the chemical reactivity that characterize doped nanocarbons. The results were published in the journal of RSC advances. E. Gracia-Espino, F. López-Urías, H. Terrones and M. Terrones. RSC Adv., 2016,6, 21954-21960 DOI: 10.1039/C5RA25278D AbstractQuantum transport studies are performed on doped and functionalized 8- and 11-armchair graphene nanoribbons (aGNRs) by means of density functional theory. Substitutional doping is performed by introducing boron, nitrogen, oxygen, silicon, phosphorus, and sulfur atoms within the lattice of the aGNRs. Other functional groups such as borane, amine, hydroxyl, thiol, silane, silene, phosphine, and phosphorane groups are also introduced at the nanoribbon's edge. The dopant position and the nanoribbon's width strongly influence the current–voltage characteristics, and generally, the narrow 8-aGNRs and edge-doped 11-aGNRs show deteriorated transport properties, mainly due to the formation of irregular edges that create highly localized states disrupting several conducting bands. On the other hand, the inside-doped 11-aGNRs are barely affected, mainly because these systems preserve the edge's structure, thus edge conduction bands still contribute to the electron transport. Our results suggest that wider graphene nanoribbons could be functionalized at the inner sections without significantly compromising their transport characteristics while retaining the chemical reactivity that characterize doped nanocarbons. Such characteristics are highly desirable in fuel cells where doped graphene is used as a catalyst support or as a metal-free catalyst.
0 Comments
Your comment will be posted after it is approved.
Leave a Reply. |
Nano for Energy group
Categories
All
Featured publications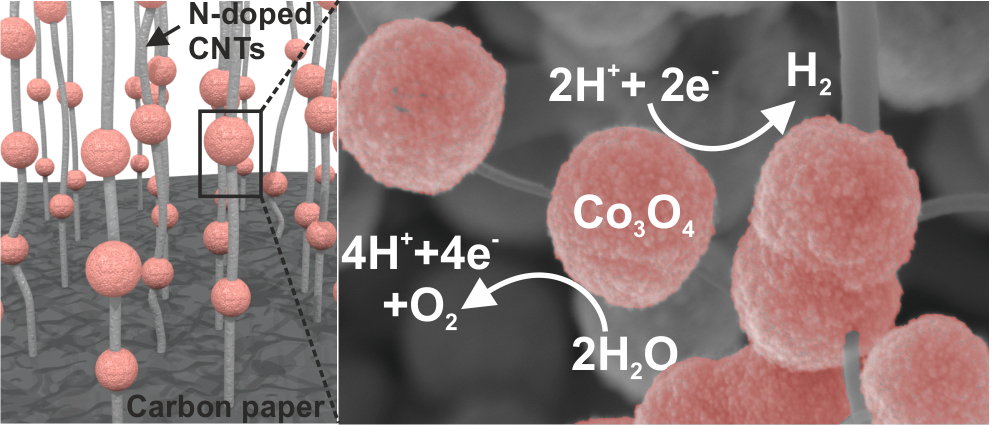
Comprehensive Study of an Earth-Abundant Bifunctional 3D Electrode for Efficient Water Electrolysis in Alkaline Medium.
ACS Appl. Mater. Interfaces, 2015, 7, 28148 
Fabrication of One-Dimensional Zigzag [6,6]-Phenyl-C61-Butyric Acid Methyl Ester Nanoribbons from Two-Dimensional Nanosheets.
ACS Nano, 2015, 9, 10516 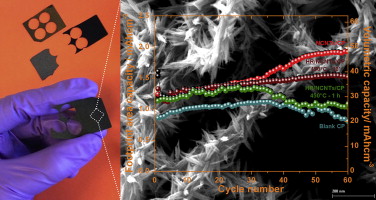
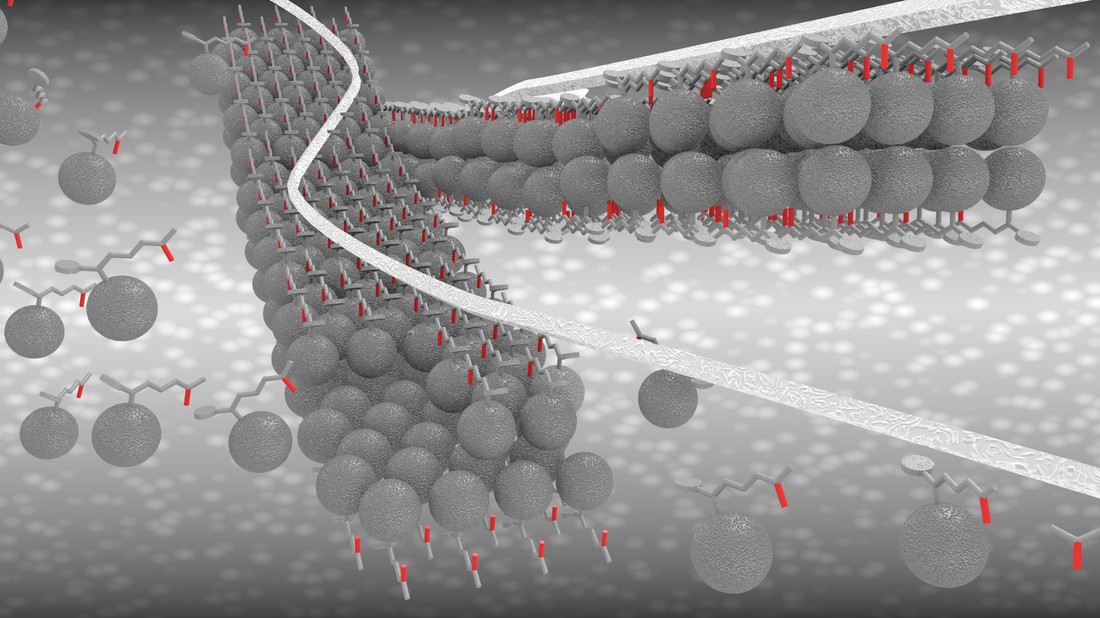
Hierarchical self-assembled structures based on nitrogen-doped carbon nanotubes as advanced negative electrodes for Li-ion batteries and 3D microbatteries.
J. P. Sources, 2015, 279, 581 .Self-Assembly Synthesis of Decorated Nitrogen-Doped Carbon Nanotubes with ZnO Nanoparticles: Anchoring Mechanism and the Effects of Sulfur.
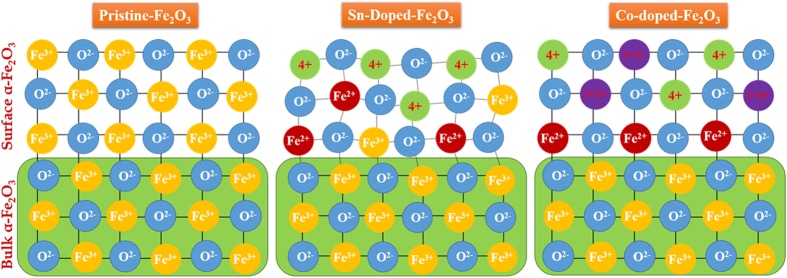
J. Phys. Chem. C, 120, 27849 (2016) Sn/Be Sequentially co-doped Hematite Photoanodes for Enhanced Photoelectrochemical Water Oxidation: Effect of Be2+ as co-dopant.
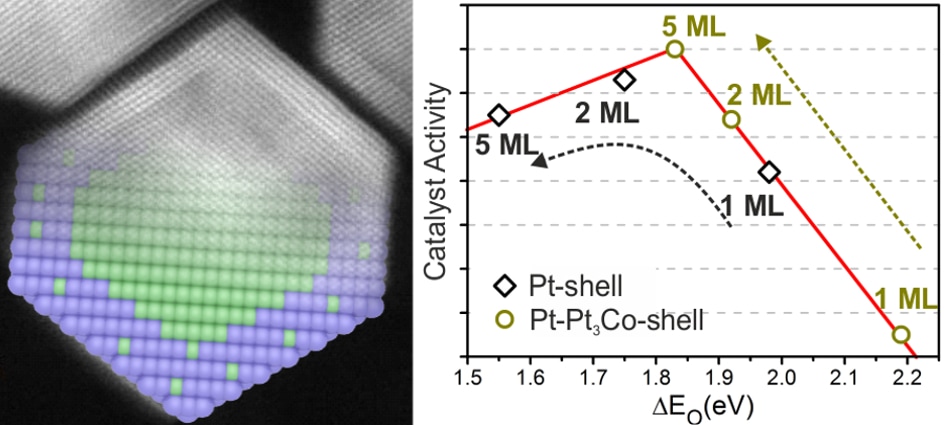
Sci Rep. 2016; 6: 23183. Atomistic understanding of the origin of high oxygen reduction electrocatalytic activity of cuboctahedral Pt3Co–Pt core–shell nanoparticles.
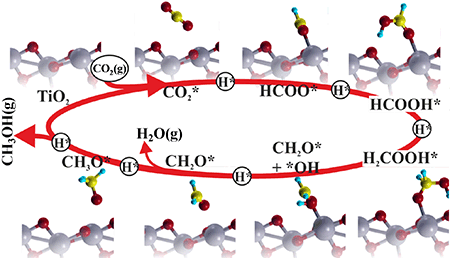
Catal. Sci. Technol., 2016, 6, 1393-1401 Photocatalytic reduction of CO2 with H2O over modified TiO2 nanofibers: Understanding the reduction pathway.
Nano Res. (2016) 9: 1956. |
 RSS Feed
RSS Feed